FODZYME® Clinical BriefUpdated 7 months ago

Introduction
An effective therapy for Irritable Bowel Syndrome (IBS) among other gastrointestinal disorders is a diet low in fermentable oligo-, di-, monosaccharides, and polyols (FODMAPs) which results in an improvement in 50-80% of patients [1].
FODMAPs are poorly absorbed short-chain carbohydrates that may drive commensal microbial gas production, promoting abdominal pain, bloating, and other symptoms related to IBS.
However, the diet is not meant to be sustained long-term, with concern for nutritional deficiencies, disordered eating, and gut microbiome changes [2].
FODZYME® is a targeted enzymatic alternative to the traditional restrictive low-FODMAP approach.
Kiwi Biosciences develops novel food supplements containing enzymes aimed at pre-digestion of FODMAPs.
In 2021, Kiwi Bio launched FODZYME®, a blend of natural enzymes featuring fructan hydrolase, an enzyme that can hydrolyze fructans (a significant contributor to FODMAP-induced symptoms).
FODZYME® also contains lactase and alpha-galactosidase aimed at the degradation of FODMAP groups lactose and galacto-oligosaccharides (GOS).
Kiwi Biosciences is on a mission to make more foods painless, with a novel polyol-targeting enzyme to address the polyol group of the FODMAP family in development.
The new solution will be transforming polyols like sorbitol and mannitol into sorbose and mannose that are more readily absorbed in the gut.
Efficacy of FODZYME® in a high-fidelity simulated gastrointestinal environment
FODZYME® was administered concurrently with 3g of inulin (a common source of fructan) in SHIME® [3], a multi-compartment scientifically validated model of the complete human gut, to study the efficacy of FODZYME®’s fructan hydrolase in fructan degradation.
Lactase and alpha-galactosidase, the two other enzymes in FODZYME®’s formulation, were assessed as an additional control arm to verify the specificity of fructan hydrolase in fructan hydrolysis.
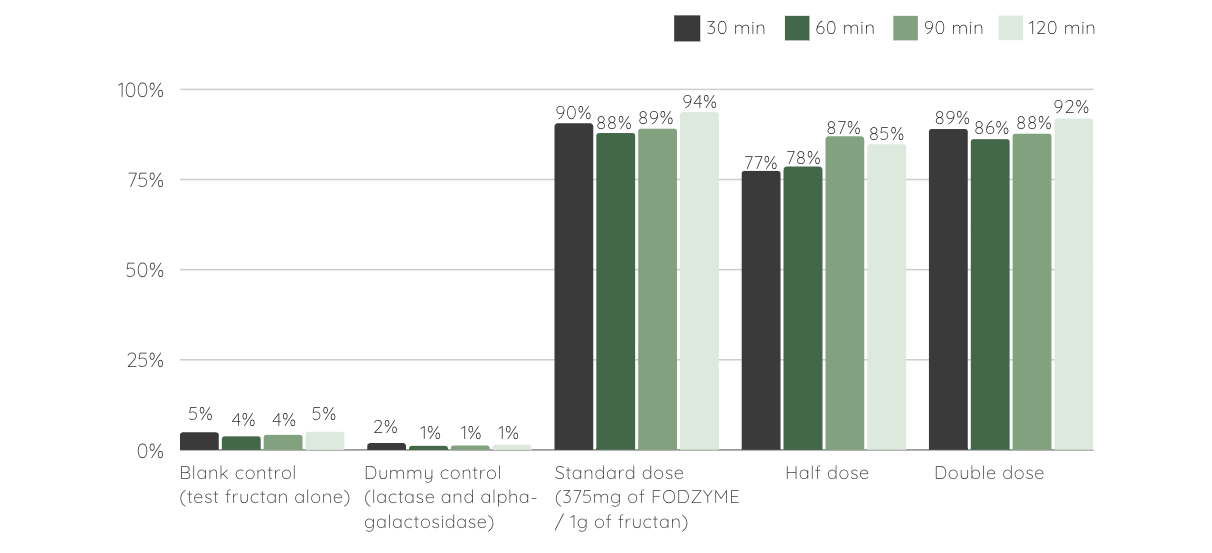
Findings indicated rapid breakdown of fructan to fructose in gastric conditions with ~90% of the inulin mass converted to fructose within 30 minutes thus demonstrating resilience to both gastric pH and protease activity.
FODZYME®'s Enzymatic Digestion in Simulated Gastric Conditions
% test fructan (3g of Inulin, Jarrow® Inulin FOS) liberated as fructose at different time points in the stomach simulator
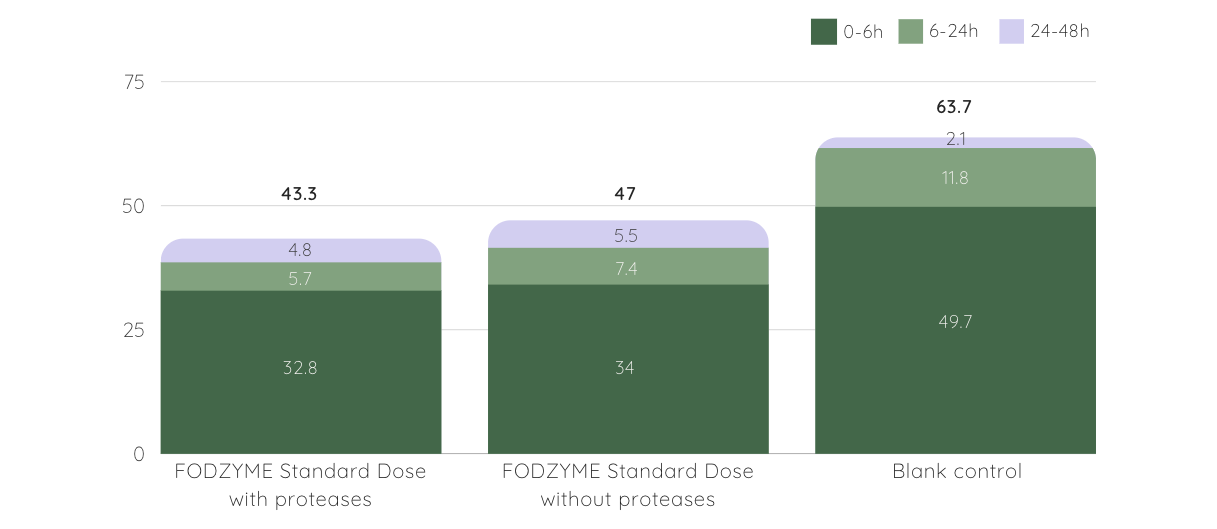
Further intestinal modeling demonstrated a significant portion of the released fructose was absorbed during simulated small intestinal transit (70%), with a corresponding decrease in microbial gas production.
FODZYME® Decreases Gas Production in Model GI Tract
▲ gas production (kPa) at different time periods
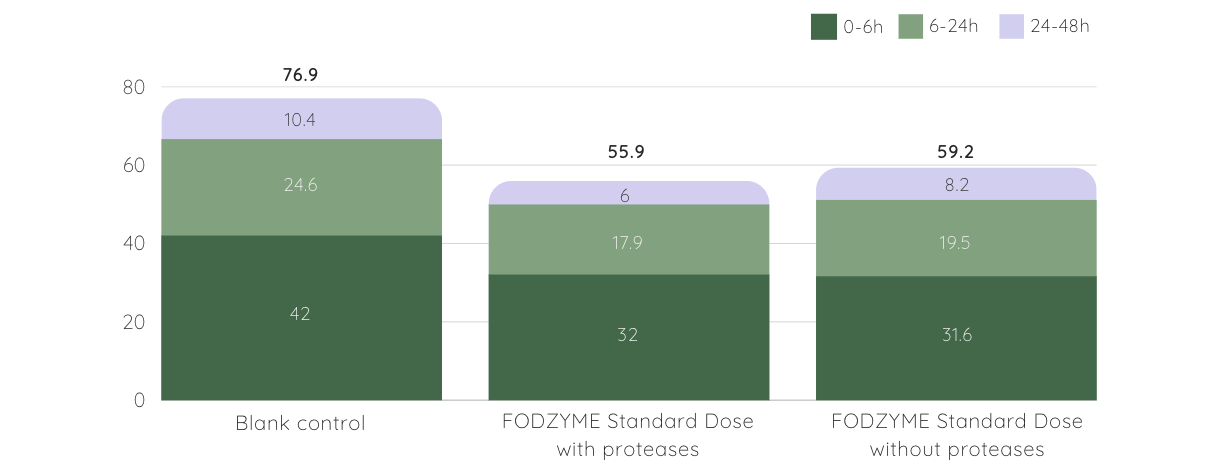
SCFA production was reduced but not depleted, suggesting FODZYME®’s enzymatic approach may be more favorable to colonic health than avoiding FODMAPs altogether [4].
Short Chain Fatty Acids (SCFA) Production is Lower but Preserved with FODZYME®
▲ total SCFA (mM) at different time periods
Preliminary in vivo efficacy
Kiwi Bio has conducted in-house blinded, randomized, placebo-controlled trials on a small group of healthy volunteers in an intensive manner.
Participants were given 40g of fructan in the form of chicory root fiber bars at once, with fructan hydrolase or placebo, and instructed to record symptoms for the next 24 hours, including every instance of flatulence along with magnitude and every bowel movement, noting a description according to the Bristol Stool Scale.
Participants were sequentially submitted to multiple interventions in a crossover style with appropriate wash-out periods in between (minimum of 48 hours between endpoints).
Kiwi Bio has also conducted extensive trials using 260g of shallots (approximately 15-30g of fructan) as substrate. Results for all types of trials are directionally similar.
However, for the purpose of maintaining fair and coherent comparisons, shown below are only results of the trials conducted each with 40g of fructan (via chicory root fiber bars):

FODZYME® Significantly Reduces Flatulence Events in Preliminary In-house Trials
Over 4x reduction in flatulence events compared to placebo (n=14, p-value 0.005) after significant ingestion of fructan (40g, about 10x the average daily consumption) and a 24hr follow-up window.
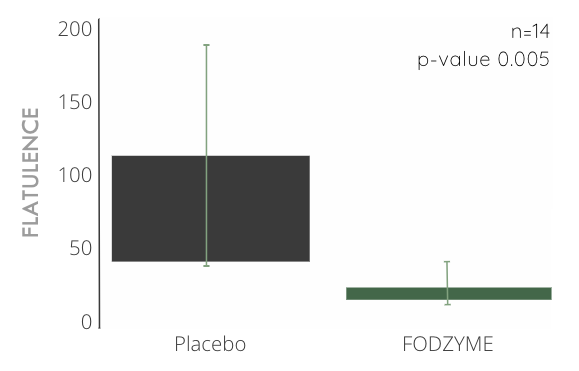
Total flatulence was weighted by magnitudes (small = 1, medium = 2, and large = 3). This likely results in a conservative estimate of FODZYME®'s effectiveness in reducing flatulence, because flatulence magnitudes were capped at "large" and participants frequently reported having "extra large" instances of flatulence during the placebo trials (and not when FODZYME® was administered).
FODZYME® Significantly Reduces Diarrhea Events in Preliminary In-house Trials
100% reduction in diarrhea events compared to placebo after significant ingestion of fructan (40g, about 10x the average daily consumption) and a 24hr follow-up window.
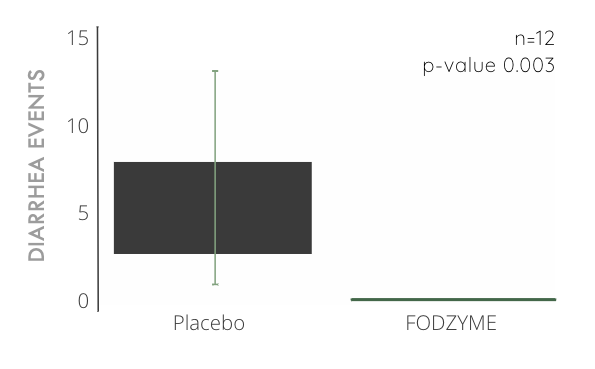
A diarrhea event is characterized by a rating of 7 on the Bristol Stool Chart.
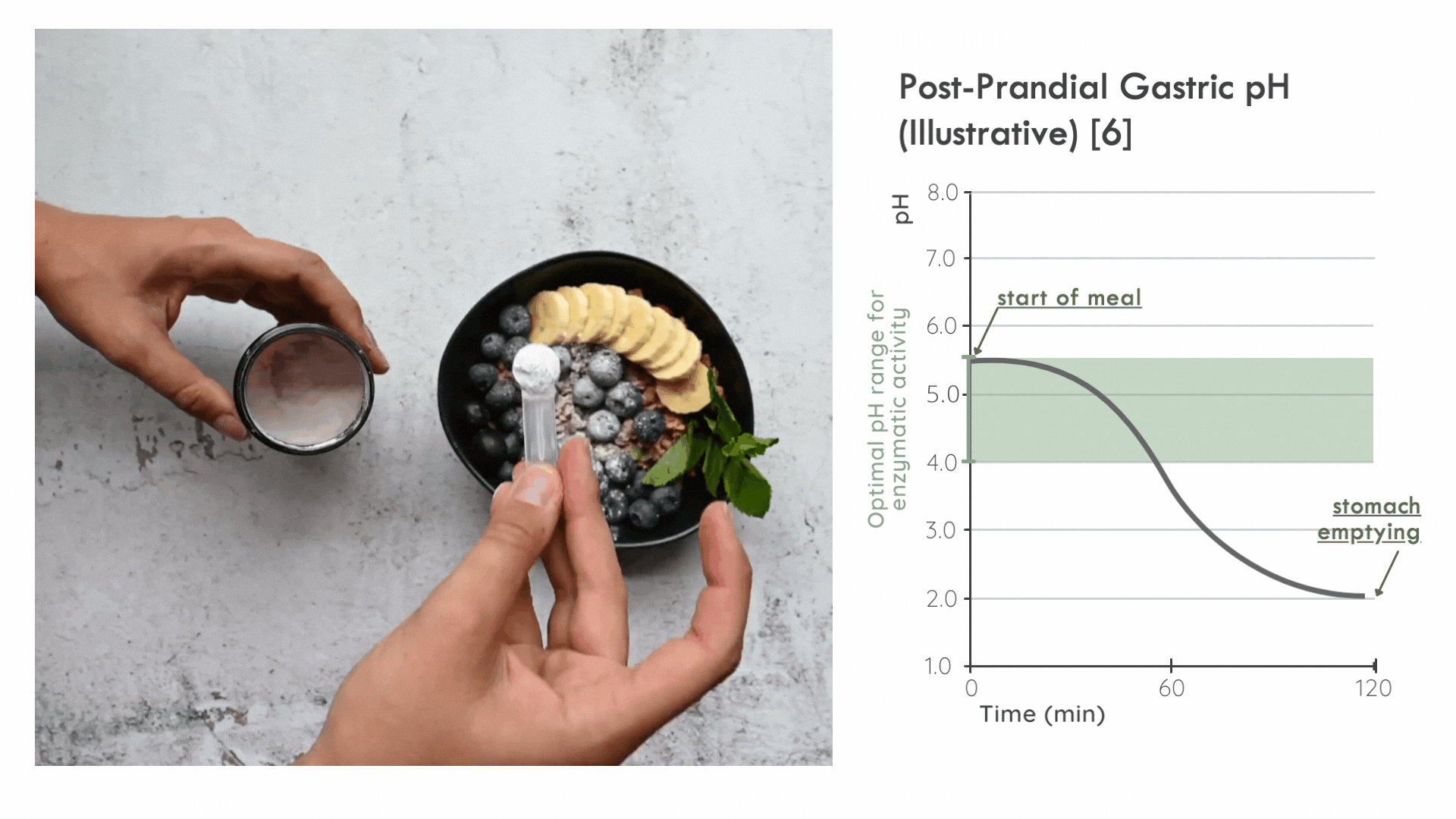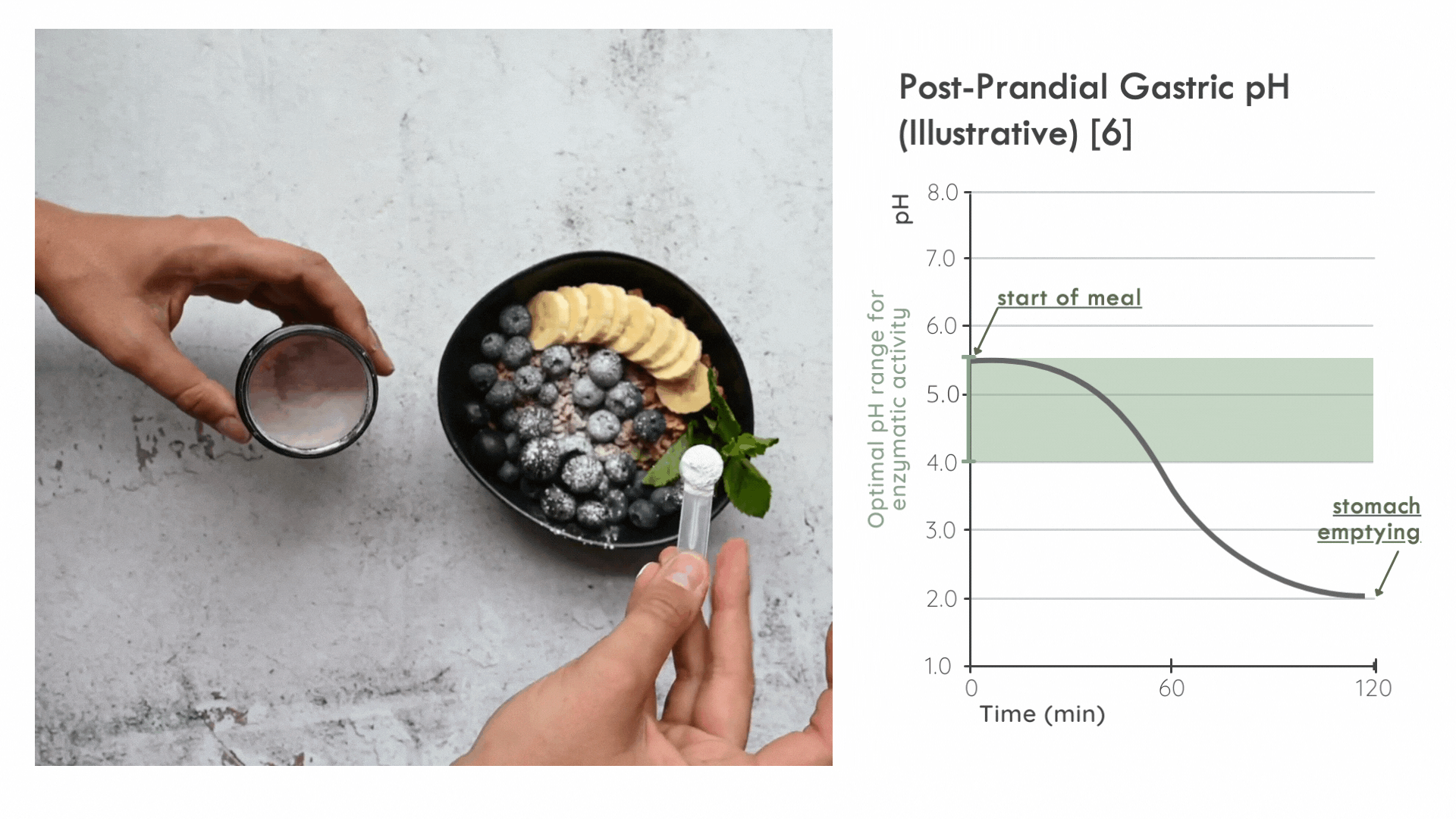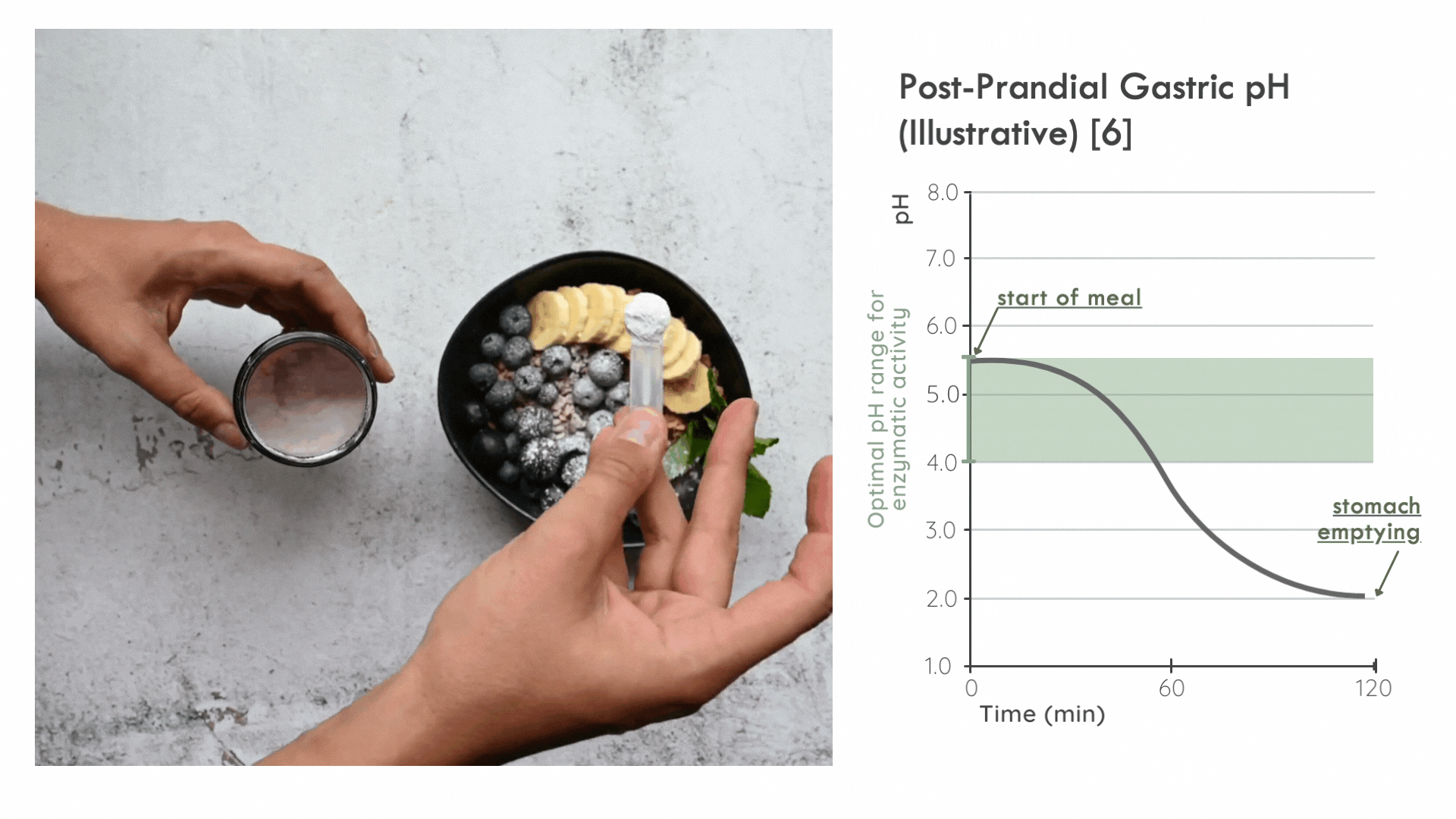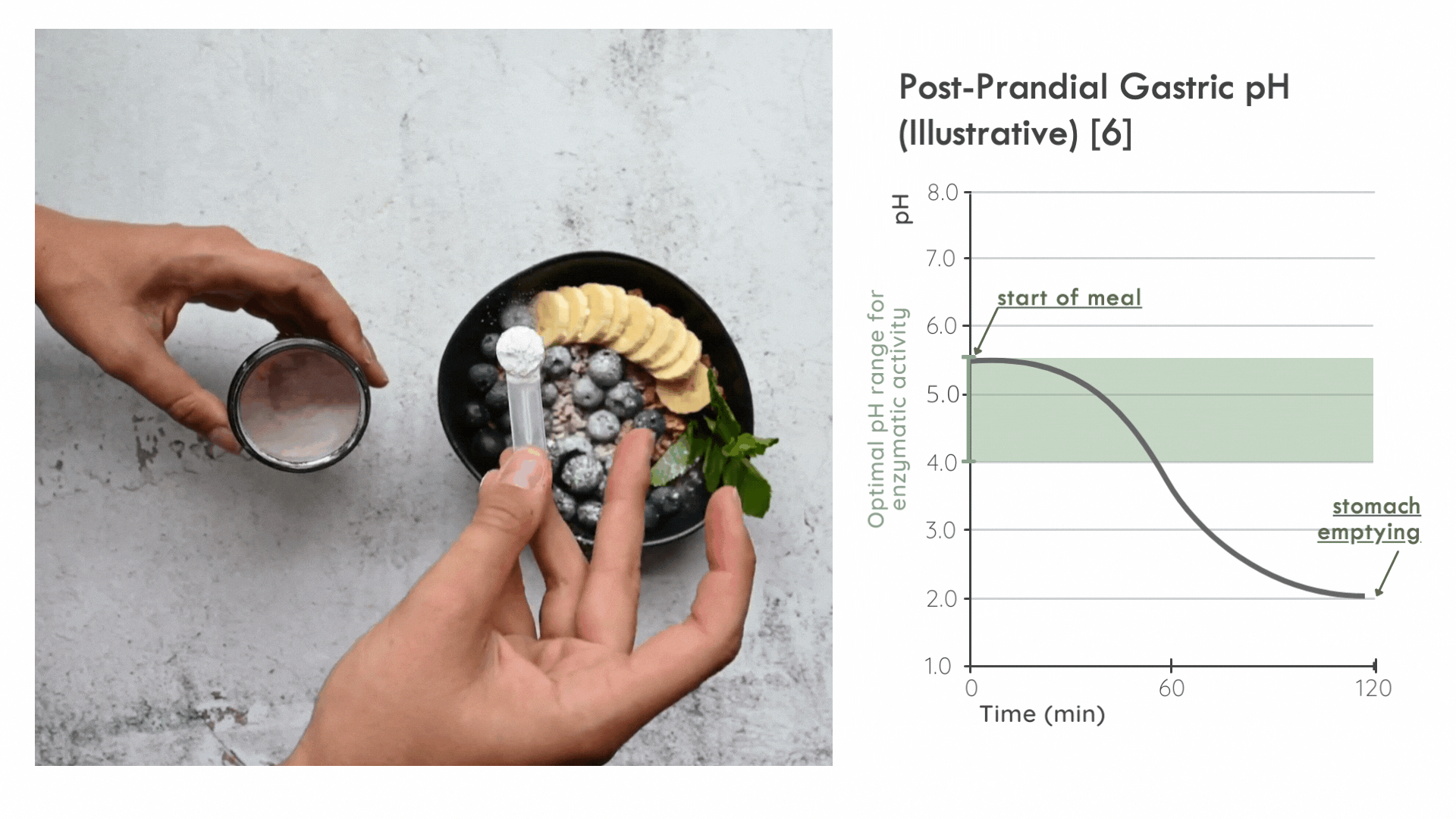
An unconventional mode of administration
Kiwi Bio has found that the overwhelming majority of digestive supplements on the market are offered as pills or capsules to be swallowed.
These are suboptimal methods of delivering the enzymes as they slow down the assimilation of enzymes with food (especially when the enzymes are encased in a wall of delayed-release cellulose).
Instead, it is critical for the enzyme to distribute in the ingested food bolus as quickly as possible to take advantage of the optimal pH range for enzymatic activity immediately following a meal. Later on, gastric pepsin becomes considerably more active and other proteases threaten enzyme stability. [5]
FODZYME® is designed as a powder to integrate directly with the meal and has been assessed in this manner throughout the SHIME experiments.
FODZYME®’s powder formulation maximizes homogenization of enzymes and food and thus the availability of enzymes to their substrates at the post-prandial gastric pH most favorable to microbial carbohydrases.
Product Safety
Fructan hydrolases are a class of enzymes that hydrolyze terminal, non-reducing 2,1- and 2,6-linked β-D-fructofuranose residues in fructans.
They have been used widely in the food processing industry for the production of fructose syrup and have been extensively documented for this use. [7][8][9]
The fructan hydrolase figuring in Kiwi Bio’s FODZYME® formulation is from an Aspergillus species source and is generally recognized as safe (GRAS) in FDA opinion letters by the FDA [10] (see table below). Note that fructan hydrolase is a carbohydrase.
Microbially derived enzymes recognized as GRAS in opinion letters from FDA
|
Carbohydrase, cellulase, glucose oxidase-catalase, pectinase, and lipase from Aspergillus niger |
|
Carbohydrase and protease from Aspergillus oryzae |
|
Carbohydrase and protease from Bacillus subtilis |
|
Invertase from edible baker's yeast or brewer's yeast (Saccharomyces cerevisiae) |
A note on fructose
Fructans are chains of fructose (and terminal glucose) and FODZYME® liberates fructose from fructan molecules.
Since fructose is itself considered a FODMAP, it is necessary to address how using the fructan hydrolase in FODZYME® will not be an issue for most fructose-sensitive individuals (except in extreme cases, such as the rare occurrence of hereditary fructose intolerance).
It is important to note that fructose is very different from the other members of the FODMAP family in that it is not the mere presence of fructose that may cause issues, but significant fructose content in excess of glucose in the meal. This is because glucose aids in the absorption of fructose for most individuals. [11]
Additionally, patient tolerance thresholds to fructose tend to be considerably higher than tolerance thresholds to oligosaccharides (arguably an order of magnitude). Clinical responders to the low-FODMAP diet, in a blinded, randomized reintroduction trial, reported significantly worse abdominal pain in response to 0.75-1.5g of fructan daily compared to 10.5-21g of fructose daily. [12]. The reintroduction of fructose in this study, despite being administered at 14 times the quantity of the fructan administered in its respective experimental arm, did not result in worse symptoms in a statistically significant manner compared with FODMAP elimination. For comparison, if FODZYME® were to degrade the entire fructan content of one clove of garlic (0.5g), this would merely introduce approx. 0.5g of extra fructose to the meal.
Moreover, most of the fructan-containing foods (e.g. garlic, onion, wheat, Brussel sprouts) themselves contain plenty of glucose or starches that break down to glucose in the body or will be consumed in dishes with plenty of glucose or glucose precursors to balance out the fructose in the digestive tract.
Hence, in typical foods that are not overly excessive in fructose, there is plenty of glucose to prevent excess fructose from being a problem. In practice, many FODZYME® users with self-reported fructose sensitivity report that they find FODZYME® highly efficacious on high-fructan meals.
About Kiwi Biosciences
Kiwi Biosciences is a human-centered biotechnology company based in Cambridge, MA devoted to developing elegant scientific solutions for extraordinary gut relief.
FODZYME® is Kiwi Bio’s first product; in development still are novel enzymes to tackle additional FODMAP groups like mannitol and sorbitol.
Kiwi Bio is led by Harvard-trained founders Anjie Liu and David Hachuel who understand firsthand how much the IBS community needs FODZYME®: Anjie as a patient herself who developed FODZYME® to be able to comfortably eat high-fructan foods and David who previously founded auggi.ai, a stool recognition AI technology and digital gut health coach for IBS patients.
They are joined by Dr. Shalaka Samant, a Yale-trained expert in microbial biotechnology, who leads Kiwi Bio’s research and development efforts.

Anjie Liu and David Hachuel,
Co-founders of Kiwi Biosciences
Our team of scientists and advisors

References
[1] Gearry R, Skidmore P, O’Brien L, Wilkinson T, Nanayakkara W. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: the evidence to date. Clinical and Experimental Gastroenterology. Published online June 2016:131. doi:10.2147/ceg.s86798
[2] Hill P, Muir JG, Gibson PR. Controversies and Recent Developments of the Low-FODMAP Diet. Gastroenterol Hepatol (N Y). 2017;13(1):36-45.
[3] Van de Wiele T, Van den Abbeele P, Ossieur W, Possemiers S, Marzorati M. The Simulator of the Human Intestinal Microbial Ecosystem (SHIME®). The Impact of Food Bioactives on Health. Published online 2015:305-317. doi:10.1007/978-3-319-16104-4_27
[4] Wong JMW, de Souza R, Kendall CWC, Emam A, Jenkins DJA. Colonic Health: Fermentation and Short Chain Fatty Acids. Journal of Clinical Gastroenterology. 2006;40(3):235-243. doi:10.1097/00004836-200603000-00015
[5] Piper, D. W.; Fenton, B. H. pH stability and activity curves of pepsin with special reference to their clinical importance. Gut 1965, 6 (5), 506–508. DOI: 10.1136/ gut.6.5.506.
[6] Dressman, J. B. et al. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm. Res. 7, 756–61 (1990).
[7] Johnson EA, Echavarri-Erasun C. Yeast Biotechnology. The Yeasts. Published online 2011:21-44. doi:10.1016/b978-0-444-52149-1.00003-3
[8] Fernandes P. Enzymatic Processing in the Food Industry. Reference Module in Food Science. Published online 2018. doi:10.1016/b978-0-08-100596-5.22341-x
[9] Singh PK, Kumar V, Yadav R, Shukla P. Bioengineering for Microbial Inulinases: Trends and Applications. Current Protein & Peptide Science. 2017;18(9). doi:10.2174/1389203718666161122112251
[10] U.S. Food and Drug Administration. Enzyme Preparations Used in Food (Partial List). Published 2020. Accessed January 11, 2022. https://www.fda.gov/food/generally -recognized-safe-gras/enzyme-preparations-used-food-partial-list
[11] Ferraris RP, Choe J, Patel CR. Intestinal Absorption of Fructose. Annual Review of Nutrition.2018;38(1):41-67. doi:10.1146/annurev-nutr-082117-051707
[12] Eswaran S, Singh P, Rifkin S, Han-Markey T, and Chey W. Are All FODMAPs Created Equal? A Blinded, Randomized Reintroduction Trial to Determine Which FODMAPs Drive Clinical Response in IBS Patients. Poster presented at: Digestive Disease Week; May 23, 2021; Virtual.
